how to find core electrons from periodic table|How do you find core and valence electrons? + Example : Tuguegarao Core electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the .
Looking for the best online sports betting sites in 2024? Discover top-rated sportsbooks offering great odds, live betting, and exciting bonuses. Bet on your favorite sports online with our comprehensive guide to the best betting sites this year. Start winning today!
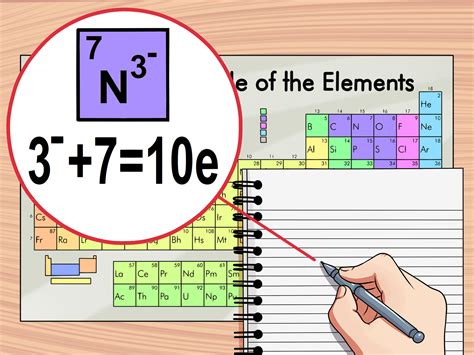
how to find core electrons from periodic table,The core electrons are in the inner shells and do not participate in chemical reactions. You can determine the number of valence electrons in the atoms of the main group elements by the group number of the element. The two electrons in the \(4s\) orbital and the seven electrons in the \(3d\) are the valence electrons: all others are core electrons. The periodicity of valance .
Identify valence electrons using the periodic table and electron configuration. Define core and valence electrons.
Figure \(\PageIndex{2}\) summarizes the type of subshell in which the distinguishing electron is to be found for atoms of elements in various regions of the .

Now that we've classified our elements into groups on the periodic table, let's see how to determine the number of valence electrons. And so for this video, we're only talking .

Core electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the .
How do you find core and valence electrons? + Example Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core . This need to gain a filled valance electron shell by having 8 valence electrons is known as the octet rule and explains why certain elements are stable or unstable despite being electrically neutral. This octet rule holds for elements in the second and third .
This chemistry video tutorial provides a basic introduction into valence electrons and the periodic table. It explains how to determine the number of valenc.
how to find core electrons from periodic table How do you find core and valence electrons? + Example 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, .
Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.The filling order is based on observed experimental results, and has been confirmed by theoretical calculations.how to find core electrons from periodic table 1. When you add 3p1, the outermost electron is in the 3p orbital (even though 3s and 3p are all valence electrons). 2. Therefore the 3p1 electron is subject to electron-electron repulsion by 3s2 electrons. 3. The distance between the nucleus and the electron in 3p is further than the 3s2 electrons.
Calculating Core Electrons. To calculate the number of core electrons in an atom, follow these steps: 1. Identify the atomic number: For a given element, look up its atomic number on the periodic table. This represents the total number of electrons in that element. 2. Determine electron configuration: Using the periodic table’s structure and .Finding the Number of Neutrons. The number of neutrons in an atom can be calculated by subtracting the atomic number from the atomic mass. Both of these numbers can be found on the periodic table. The atomic number is listed above the symbol of the element whereas the mass number is placed below. Let’s keep using oxygen as our example. Summary. Electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of inner-shell electrons. Electrons in an s s orbital can shield p p electrons at the same energy level because of the spherical shape of the s s orbital. Electrons in paired spin configurations are slightly easier to .Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 3.1.6. 3.1. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:
And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system for classifying groups. So one, two, three, four, five, six, seven, and eight. So we're going to ignore the other way to number the groups.Microsoft Teams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors.
This chemistry video tutorial provides a basic introduction into valence electrons and the periodic table. It explains how to determine the number of valenc. Useful Relationships from the Periodic Table. The periodic table of elements is useful in determining the charges on simple monoatomic ions. For main-group elements, those categorized in . Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 3.4.6 3.4. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series:Ionization Energies of s- and p-Block Elements. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(Li\) and \(Be\) (Table \(\PageIndex{2}\)): successive ionization energies increase steadily as electrons are removed from the valence orbitals (3s or 3p, in this case), followed by an especially .
Figure 9.4.4 9.4. 4 graphs the relationship between the first ionization energy and the atomic number of several elements. Within a period, the values of first ionization energy for the elements (IE 1) generally increases with increasing Z. Down a group, the IE 1 value generally decreases with increasing Z.
Their electron configurations are 1 s1 and 1 s2, respectively; with He, the n = 1 shell is filled. These two elements make up the first row of the periodic table (see Figure 8.9 “The 1s Subshell”). Figure 8.9 The 1s Subshell. The next two electrons, for Li and Be, would go into the 2s subshell.
Electron configurations can be predicted by the position of an atom on the periodic table. 9.7: Electron Configurations and the Periodic Table is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The arrangement of electrons in atoms is responsible for the shape of the periodic table.
how to find core electrons from periodic table|How do you find core and valence electrons? + Example
PH0 · Valence electrons (video)
PH1 · Valence Electrons and the Periodic Table
PH2 · How do you find core and valence electrons? + Example
PH3 · Determine valence electrons using the periodic table
PH4 · Counting valence electrons for main group elements
PH5 · Core electron
PH6 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH7 · 5.17: Electron Configurations and the Periodic Table
PH8 · 3.4: Core and Valence Electrons
PH9 · 1.9B: Valence and Core Electrons